
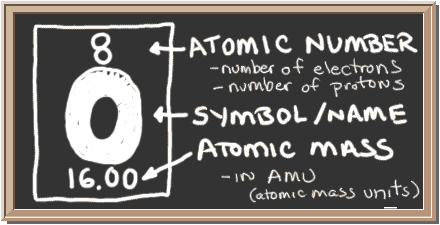
protons are small, positively charged particles with, by definition, a mass of one unit (one a.m.u.).There are three types of subatomic particles that are found in the atoms of all but one element. the physical and chemical properties of all elements are the result of the origination of substructure of their atoms.the unique chemical properties of just a few atoms determine the form and function of all living things.The three most abundant elements on earth are oxygen, silicon and aluminum in living organisms the six most abundant elements are carbon, hydrogen, nitrogen, oxygen, phosphorus and sulfur. there are 92 natural elements and 13 more that have been created in a laboratory.all the atoms in a pure substance or element are identical to one another but different to the atoms in a different element.each element is made up of identical particles of matter called atoms.a pure or elementary substance cannot be broken down into smaller or simpler constituents without loosing all its original properties such pure substances are called elements.common substances, such as air, water, wood and cloth are mixtures of materials and can be chemically or physically broken down into simpler substances.
Make sure that you have written down the following definitions, explanations and important concepts in your notes.Īn atom is the smallest unit of a pure substance or element that can exist and still retain the properties of the original substance or element. Use this section to check up on the accuracy of your lecture notes.

Available here Image Courtesy:ġ.’Electron shell 008 Oxygen – no label’By Pumbaa (original work by Greg Robson) (CC BY-SA 2.0 uk) via Commons WikimediaĢ.’Figure 02 01 09’By CNX OpenStax, (CC BY 4.Science at a Distance Science at a Distance “The Differences of Oxygen & Oxygen Gas.” Sciencing, 24 Apr. “Allotropes of Oxygen.” Wikipedia, Wikimedia Foundation. The difference between atomic oxygen and molecular oxygen is that atomic oxygen is highly reactive and does not exist in the atmosphere as it is whereas molecular oxygen is less reactive and exists in the atmosphere as it is. Summary – Atomic Oxygen vs Molecular OxygenĪtomic and molecular oxygen are chemical species derived from the chemical element, oxygen which has the atomic number 8. It exists as itself in our atmosphere (about 21%). Molecular oxygen is diatomic oxygen which has the symbol O 2. It does not exist naturally for even a short period, but in outer space, it is the predominant form of oxygen. What is the Difference Between Atomic Oxygen and Molecular Oxygen?Ītomic oxygen is a very reactive chemical species having the symbol O(3P). Therefore, this atom does not exist naturally for even a short period it tends to react with other chemical elements or compounds to become stable by pairing its unpaired electron. This means atomic oxygen has an unpaired electron that makes this atom highly reactive.

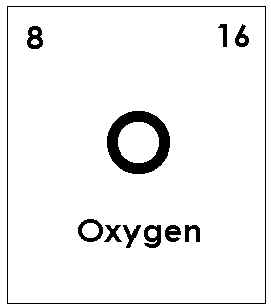
Side by Side Comparison – Atomic Oxygen vs Molecular Oxygen in Tabular FormĪtomic oxygen is a very reactive chemical species having the symbol O(3P). Therefore, it cannot exist as an individual chemical species because of its high reactivity. It has two oxygen atoms bonded to each other via covalent bond. But when we refer to oxygen in common use, we are talking about molecular oxygen that we breathe. Oxygen is a chemical element having the atomic number 8.
#Oxygen atomic number free#
Moreover, atomic oxygen is a free radical having the symbol O(3P) while the molecular oxygen is a diatomic oxygen having the symbol O 2. The key difference between atomic oxygen and molecular oxygen is that the atomic oxygen is highly reactive and does not exist in the atmosphere as it is whereas the molecular oxygen is less reactive and exists in the atmosphere as it is.


 0 kommentar(er)
0 kommentar(er)
